How many moles of H2S are produced? This question lies at the heart of understanding the stoichiometry and mole calculations involved in the production of hydrogen sulfide (H2S). This comprehensive guide delves into the intricacies of moles, balanced chemical equations, and stoichiometry to provide a thorough examination of H2S production.
The concept of moles serves as a fundamental unit for quantifying the amount of a substance in chemistry. By utilizing balanced chemical equations, we can determine the stoichiometry of reactions, allowing us to calculate the moles of H2S produced from a given quantity of reactants.
Understanding the Concept of Moles
A mole is a fundamental unit of measurement in chemistry that represents a specific quantity of a substance. It is defined as the amount of a substance that contains exactly 6.02214076 × 10 23elementary entities, such as atoms, molecules, ions, or electrons.
The mole concept plays a crucial role in chemistry, allowing scientists to determine the number of particles present in a given sample and perform quantitative calculations involving chemical reactions.
Examples of Mole Usage
- 1 mole of carbon contains 6.02214076 × 10 23carbon atoms.
- 1 mole of water (H 2O) contains 6.02214076 × 10 23water molecules.
- 1 mole of sodium chloride (NaCl) contains 6.02214076 × 10 23sodium ions and 6.02214076 × 10 23chloride ions.
Balanced Chemical Equations
A balanced chemical equation is a representation of a chemical reaction that shows the exact number of atoms or molecules of each reactant and product involved. Balancing chemical equations ensures that the number of atoms of each element on the reactants’ side equals the number of atoms of the same element on the products’ side.
This principle is based on the law of conservation of mass, which states that matter cannot be created or destroyed in a chemical reaction.
Importance of Balanced Chemical Equations
- Provide a quantitative understanding of chemical reactions.
- Allow for the determination of the stoichiometry of reactions, which is the relative amounts of reactants and products involved.
- Enable predictions about the amount of product that can be obtained from a given amount of reactants.
Stoichiometry and Mole Calculations: How Many Moles Of H2s Are Produced
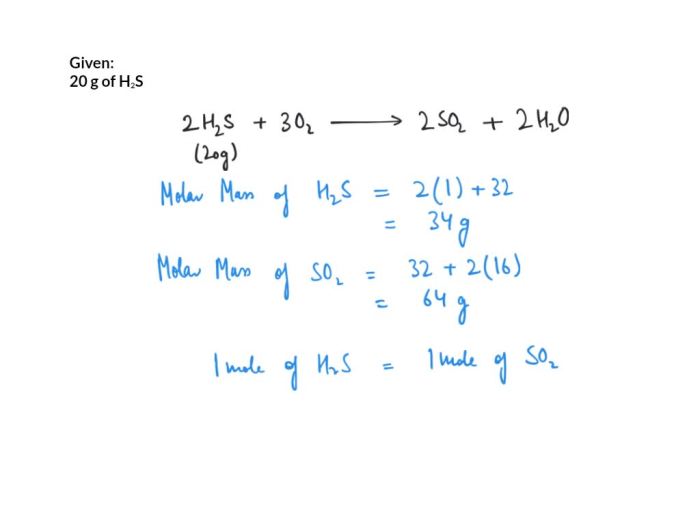
Stoichiometry is the branch of chemistry that deals with the quantitative relationships between reactants and products in chemical reactions. Mole calculations are a fundamental aspect of stoichiometry and involve converting between the mass of a substance and its corresponding number of moles.
Examples of Mole Calculations
- Calculating the number of moles of sodium chloride (NaCl) in 58.44 g of NaCl:
- Calculating the mass of 0.5 moles of water (H 2O):
“` Moles of NaCl = Mass of NaCl / Molar mass of NaCl Moles of NaCl = 58.44 g / 58.44 g/mol Moles of NaCl = 1 mol “`
“` Mass of H 2O = Moles of H 2O × Molar mass of H 2O Mass of H 2O = 0.5 mol × 18.015 g/mol Mass of H 2O = 9.008 g “`
Specific Case: Hydrogen Sulfide (H2S) Production
Hydrogen sulfide (H 2S) is a colorless, flammable, and toxic gas with a characteristic “rotten egg” odor. It is produced naturally by anaerobic bacteria and can also be generated industrially.
Balanced Chemical Equation for H2S Production, How many moles of h2s are produced
“`
H2+ S → 2 H 2S
“`
This equation shows that 2 moles of hydrogen gas (H 2) react with 1 mole of sulfur (S) to produce 2 moles of hydrogen sulfide (H 2S).
Stoichiometry of H2S Production
Using the balanced chemical equation, we can determine the stoichiometry of the reaction. For every 2 moles of H 2consumed, 1 mole of S is consumed, and 2 moles of H 2S are produced.
Examples and Applications
The following table shows examples of different amounts of reactants and the corresponding moles of H 2S produced:
| Hydrogen Gas (H2) | Sulfur (S) | Hydrogen Sulfide (H2S) |
|---|---|---|
| 1 mol | 0.5 mol | 1 mol |
| 2 mol | 1 mol | 2 mol |
| 4 mol | 2 mol | 4 mol |
H 2S production has various industrial applications, including:
- As a precursor for the production of sulfuric acid, which is used in a wide range of industries, including fertilizer production, petroleum refining, and metal processing.
- In the production of dyes, pigments, and other chemicals.
- As a fuel in certain industrial processes.
Clarifying Questions
What is the definition of a mole?
A mole is the SI unit of amount of substance, defined as the amount of substance that contains as many elementary entities as there are atoms in 0.012 kilograms of carbon-12.
How do I use a balanced chemical equation to determine the moles of H2S produced?
By analyzing the coefficients in a balanced chemical equation, you can establish the mole ratios between the reactants and products. This enables you to calculate the moles of H2S produced based on the known moles of reactants.