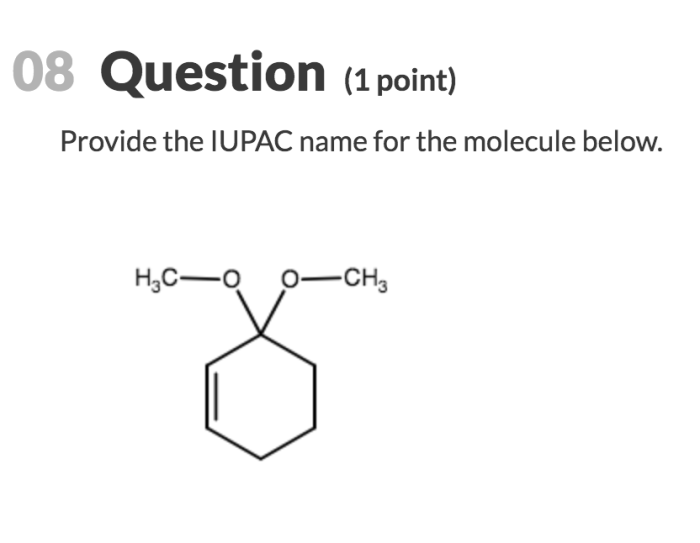
Provide the iupac name for the molecule below. – Delving into the realm of IUPAC nomenclature, this guide embarks on an enlightening journey to decipher the systematic naming of organic compounds. Embracing the principles established by the International Union of Pure and Applied Chemistry (IUPAC), we delve into the intricacies of identifying functional groups, selecting parent chains, numbering carbon atoms, and assigning locants to substituents.
Through a comprehensive exploration of IUPAC rules and their application, this guide empowers readers to confidently navigate the complexities of organic compound nomenclature.
IUPAC Nomenclature Basics
IUPAC nomenclature is a systematic method of naming organic compounds. It is based on the principles of simplicity, uniqueness, and general applicability.
The basic rules of IUPAC nomenclature are as follows:
- The parent chain is the longest continuous carbon chain in the molecule.
- The substituents are named in alphabetical order.
- The locants of the substituents are the numbers of the carbon atoms to which they are attached.
- The prefixes “di”, “tri”, “tetra”, etc., are used to indicate the number of identical substituents.
Identifying Functional Groups
Functional groups are atoms or groups of atoms that are responsible for the characteristic chemical properties of a molecule.
The following table lists some common functional groups and their corresponding prefixes and suffixes:
| Functional Group | Prefix | Suffix |
|---|---|---|
| Alkyl | alkyl- | -ane |
| Alkenyl | alkenyl- | -ene |
| Alkynyl | alkynyl- | -yne |
| Alcohol | hydroxy- | -ol |
| Aldehyde | oxo- | -al |
| Ketone | oxo- | -one |
| Carboxylic acid | carboxy- | -oic acid |
Parent Chain Selection
The parent chain is the longest continuous carbon chain in the molecule.
To select the parent chain, follow these steps:
- Identify the longest carbon chain in the molecule.
- If there is more than one chain of equal length, choose the chain that contains the most double or triple bonds.
- If there is still more than one chain of equal length, choose the chain that has the most substituents.
Numbering the Parent Chain
The parent chain is numbered to give the substituents the lowest possible locants.
To number the parent chain, follow these steps:
- Start numbering at the end of the chain that is closest to the substituent.
- If there are two or more substituents, number the chain so that the sum of the locants is the lowest possible.
Naming Substituents
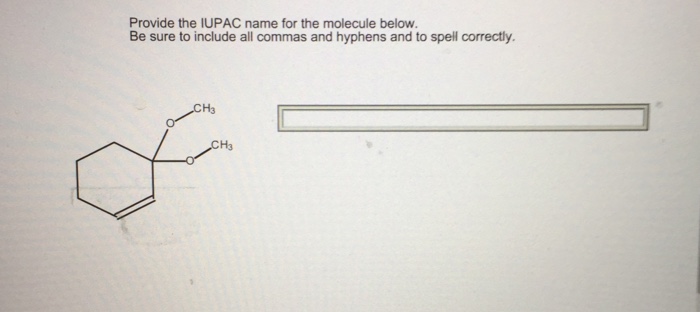
Substituents are named by their parent hydrocarbon group, followed by the appropriate suffix.
The following table lists some common substituents and their corresponding names:
| Substituent | Name |
|---|---|
| -CH3 | methyl |
| -CH2CH3 | ethyl |
| -CH=CH2 | vinyl |
| -C≡CH | ethynyl |
| -OH | hydroxy |
| -CHO | formyl |
| -COOH | carboxy |
Multiple Substituents
When a molecule has multiple substituents, the prefixes “di”, “tri”, “tetra”, etc., are used to indicate the number of identical substituents.
For example, a molecule with two methyl substituents is named 2-methylpropane.
Branched Structures
Branched structures are named by identifying the parent chain and the substituents on the parent chain.
To name a branched structure, follow these steps:
- Identify the parent chain.
- Identify the substituents on the parent chain.
- Name the substituents.
- Number the parent chain to give the substituents the lowest possible locants.
- Combine the names of the substituents and the parent chain to form the name of the branched structure.
Functional Group Priority
In IUPAC nomenclature, functional groups have priority over other substituents.
The following table lists some common functional groups in order of decreasing priority:
| Functional Group | Priority |
|---|---|
| Carboxylic acid | 1 |
| Ester | 2 |
| Amide | 3 |
| Aldehyde | 4 |
| Ketone | 5 |
| Alcohol | 6 |
| Alkene | 7 |
| Alkyne | 8 |
Complex Molecules: Provide The Iupac Name For The Molecule Below.
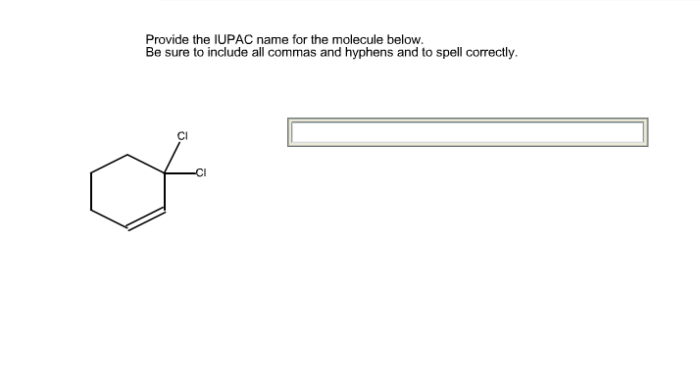
IUPAC nomenclature can be used to name even the most complex molecules.
To name a complex molecule, follow these steps:
- Identify the parent chain.
- Identify the substituents on the parent chain.
- Name the substituents.
- Number the parent chain to give the substituents the lowest possible locants.
- Combine the names of the substituents and the parent chain to form the name of the complex molecule.
Essential FAQs
What is the significance of IUPAC nomenclature?
IUPAC nomenclature provides a standardized system for naming organic compounds, ensuring consistency and clarity in scientific communication. It facilitates the unambiguous identification and description of complex molecular structures, enabling chemists to accurately convey chemical information.
How do I determine the parent chain in IUPAC nomenclature?
To determine the parent chain, identify the longest continuous carbon chain within the molecule. This chain serves as the foundation for assigning locants to substituents and determining the base name of the compound.
What is the role of functional groups in IUPAC naming?
Functional groups are specific atomic arrangements within a molecule that impart characteristic chemical properties. Identifying functional groups is crucial in IUPAC nomenclature as they determine the suffix of the compound name and influence the priority rules for naming.