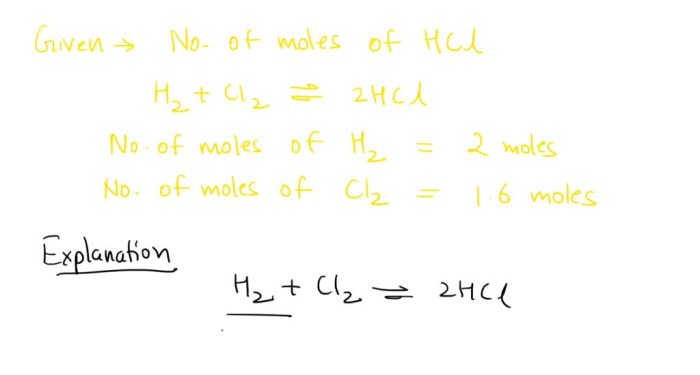Aspirin synthesis pre lab answers – Aspirin synthesis pre-lab answers provide a comprehensive understanding of the preparation and execution of the aspirin synthesis experiment. By delving into the background, safety protocols, experimental procedures, data analysis, and factors affecting the synthesis, this guide equips students with the knowledge and skills necessary to successfully conduct this fundamental chemistry experiment.
Aspirin, a widely used over-the-counter medication, serves as a prime example of the practical applications of organic chemistry. The aspirin synthesis experiment offers a hands-on approach to understanding the principles of organic synthesis, emphasizing the importance of safety, precision, and critical thinking.
Aspirin Synthesis: Background and Overview
Aspirin, known by its chemical name acetylsalicylic acid, is a widely used over-the-counter medication with a rich history in medicine. Its origins can be traced back to ancient times, with records indicating its use as a pain reliever in the form of willow bark extract.
The isolation and synthesis of pure aspirin in the late 19th century marked a significant advancement in the treatment of pain, fever, and inflammation.
Chemically, aspirin is an ester of salicylic acid and acetic acid. It possesses a unique structure characterized by an aromatic ring, a carboxylic acid group, and an acetyl group. Aspirin’s pharmacological properties stem from its ability to inhibit the enzyme cyclooxygenase (COX), which plays a crucial role in the synthesis of prostaglandins, mediators of pain, inflammation, and fever.
Pre-Lab Preparation: Safety and Materials
Safety is paramount when handling chemicals in the laboratory. Before embarking on the aspirin synthesis experiment, it is essential to familiarize oneself with the potential hazards and appropriate safety precautions. These include wearing appropriate protective gear such as gloves, lab coats, and safety glasses, and working in a well-ventilated area.
The following materials are required for the aspirin synthesis experiment:
- Salicylic acid
- Acetic anhydride
- Concentrated sulfuric acid
- Distilled water
- Round-bottom flask
- Condenser
- Thermometer
- Separatory funnel
- Filter paper
- Büchner funnel
- Vacuum filtration apparatus
Experimental Procedures: Step-by-Step Guide
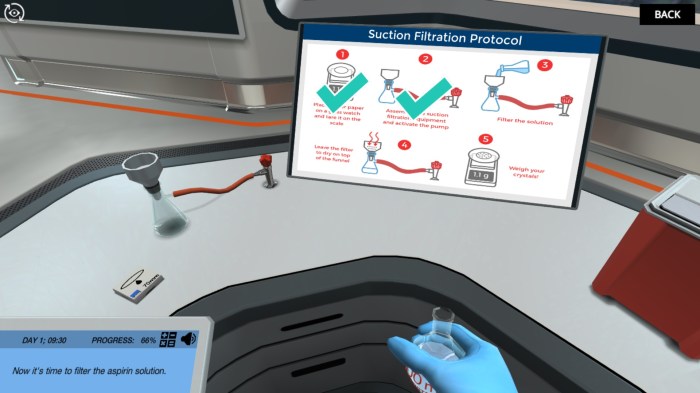
The aspirin synthesis experiment involves a series of steps that must be followed carefully to ensure safety and successful synthesis. Here is a detailed guide to the experimental procedure:
- Reaction Setup:In a round-bottom flask, combine salicylic acid, acetic anhydride, and a few drops of concentrated sulfuric acid. Equip the flask with a condenser and thermometer.
- Heating and Reaction:Heat the reaction mixture gently using a heating mantle or hot plate. Monitor the temperature closely and maintain it between 130-140°C for approximately 30 minutes.
- Cooling and Crystallization:After the reaction is complete, allow the mixture to cool to room temperature. Slowly add distilled water to the flask to induce crystallization.
- Filtration:Filter the crystals using a Büchner funnel and vacuum filtration apparatus. Wash the crystals thoroughly with distilled water.
- Drying:Spread the crystals on a filter paper and allow them to dry in a well-ventilated area or oven at a low temperature.
Essential FAQs: Aspirin Synthesis Pre Lab Answers
What is the purpose of the aspirin synthesis pre-lab?
The aspirin synthesis pre-lab provides essential information and instructions for the successful execution of the aspirin synthesis experiment, ensuring safety, accuracy, and efficient use of time in the laboratory.
What safety precautions should be taken during the aspirin synthesis experiment?
Proper safety precautions include wearing appropriate personal protective equipment, handling chemicals with care, and following all experimental procedures as Artikeld in the pre-lab.
How is the yield of aspirin calculated?
The yield of aspirin is calculated by comparing the mass of the purified aspirin obtained to the theoretical yield, which is based on the stoichiometry of the reaction.